An innovative way to store vaccines (e.g. Covid 19)
at low temperatures
It was the end of 2020. The Covid-19 pandemic is spreading around the world. There is an announcement that Pfizer is preparing to produce and distribute next-generation vaccines. New generation vaccines that require them to be kept at a temperature of -75°C (CO2 sublimation temperature) during distribution and storage.
Since vaccination was to cover the whole world, I was asked whether it was possible to build a refrigerated device for storing vaccines that met the following requirements:
– maintain the set storage temperature despite long breaks of many hours in the supply of electricity,
– allows for smooth selection of storage temperature from ambient to temperature – 90°C,
– in the event of power outages or device failure, the temperature of the vaccine will not be increased by more than 10°C in a period of 48 hours.
– capacity: two containers of PFIZER vaccines @ 10,000 pcs.
– autonomy of storage: not shorter than two weeks,
– refrigerant: ecological – preferably natural,
– simple design,
-simple service requiring neither specialized equipment nor specialist knowledge.
-possibility of cooperation with renewable energy sources (photovoltaics, windmills)
To sum up. The storage device is intended to be a sustainable solution, adapted to the storage of vaccines at low temperatures in areas with an unstable electricity grid and where there is no highly specialized service.
I started my work by familiarizing myself with the devices offered on the market for storing small products at temperatures below -50°C. It turned out that the following types of refrigeration equipment are available on the market:
– Cascade type. The unit consists of a low-temperature chiller with R23 refrigerant (GWP = 14,800 ), the condenser of which is cooled by a typical refrigeration device ( with R404A refrigerant) .
– Modified refrigeration unit with single single-stage compressor and EP88 refrigerant (GWP = 4425) or ethane (both flammable).
The disadvantages of the above solutions are:
– use of refrigerant with a high GWP (Global Warming Potential) or flammable (butane, propane, ethylene).
– highly specialized service equipped with specialized equipment and special spare parts.
– the need to secure the continuity of electrical supply.
– significant electricity consumption during operation.
The above-mentioned inconveniences made it necessary to look for an innovative technical solution.
Solution concept for an innovative device for low-temperature storage of vaccines
In order to meet the requirements, I decided to use the well-known technique and technologies of storing products using LN2 by modifying it.
The advantages of using LN2 as a refrigerant are:
– fully ecological (simply perfect): non-flammable, non-toxic, GWP=0,
– universal availability (79% content is in the atmosphere),
– ease of its production (liquefaction) with the use of typical equipment – figure 1,
– ease of distribution.

Figure 1. Liquid nitrogen (LN2) liquefaction plant with capacity of 120 kg/day with a 200 liter tank. Energy requirement: 13 kW. (Stirling Cryogenics)
Individual COVID-19 vaccine storage device.
The idea of building the device was based on the design of a typical tank for storing liquefied nitrogen (LN2). The tank, in order to maintain a temperature of minus 200 °C, „consumes” a certain amount of liquid nitrogen. „Wear” consists in its partial evaporation. The heat required to evaporate LN2 is equal to the heat penetrating through the tank insulation. A by-product of LN2 „consumption” is nitrogen vapor at a temperature of -200 °C .
A typical LN2 tank, in order to maintain a temperature of -200 °C, „consumes” a certain amount of liquid nitrogen. „Wear” consists in its partial evaporation. The heat required to evaporate LN2 is equal to the heat penetrating through the insulation of the tank. Cold nitrogen vapours in conventional LN2 tanks are released to the outside via a safety valve. The idea of using the „cold” contained in the vapours created in this way and using them as a cooling agent for the device was the basis for the construction of a vaccine storage device. In order to minimize the need for cooling, it was decided to construct the device in accordance with the LN2 tank technology (MLI insulation), and to protect against not exceeding the temperature increase of more than 10 . C during 48 hours in case of failure, a spirit tank was installed as a cold storage. By placing a nitrogen coil in a spirit tank, this tank cools the vaccine storage space and provides cooling in case of an emergency. The tank with the nitrogen coil as well as the entire device are shown in Figures 2 and 3.
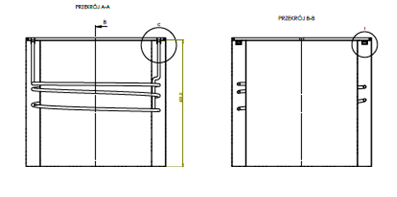
. Figure 2. Cold storage (accumulator – (V=20 L of spirit tank with nitrogen coil)
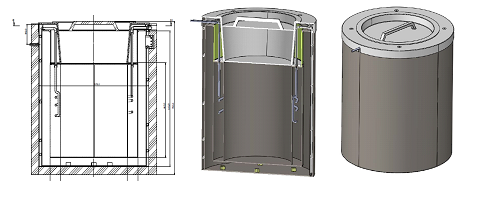
. Figure 3. COVID – 19 vaccine storage device.
Energy consumption by the controller and solenoid valves can be carried out directly from the mains or battery. The battery is selected in such way that in the event of a power outage, it will be able to maintain the operation of the device for 48 hours. If the supply of electricity is resumed, the battery is „regenerated” within one hour. In the event of a further lack of power, after the battery is exhausted, the device goes into emergency operation using the „cool” battery and sending an alarm signal for supervision and service.
In order to meet the requirement of 48 hours of maintaining the proper storage temperature despite the lack of electrical power or failure of the device, it was decided to use a „cold” battery in the form of a container with spirit (methyl alcohol with a freezing point of – 110°C)
On the basis of the assumed value of the „k” factor of the tank, the dimensions of the container with vaccines, the capacity of the thermal accumulator was determined 20 liters. A „nitrogen” coil was assembled to regulate the storage temperature in the cold battery. This coil is supplied from one end with nitrogen vapors or liquid nitrogen, depending on the needs. Leaving the coil, the partially heated nitrogen vapors are directed to the space between the outer wall of the storage tank and the additional external insulation. The external space of the storage tank is therefore cooled, which reduces its heat load on the storage tank. The diagram of the nitrogen installation shows figure 4.
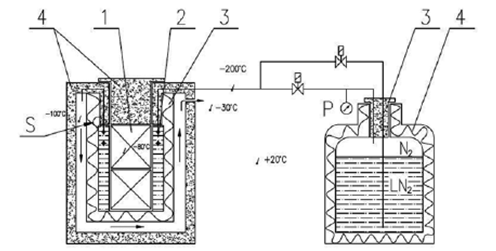
Figure 4. Innovative Covid -19 vaccine storage device. 1- container with vaccines (@ 10 000); 2 – nitrogen coil with cold storage; 3- insulation MLI; 4- polyurethane insulation
During the tests of the demonstrator, it turned out that the structural solution used to connect the internal and external flanges (by means of gluing) did not work and did not ensure that the appropriate vacuum was maintained. As a result, the „k” factor deteriorated, which increased nitrogen consumption above the assumed value. Temperature variation during the demonstrator test is shown in Figure 5.

Figure 5. Demonstrator Study. Storage temperature – green color
The results obtained indicate that the control system and control algorithm used worked correctly. The device maintained the assumed storage temperature of -35°C and -60°C and reached the assumed temperature of -90°C. However, the low k-value of the insulation resulted in rapid „consumption” of liquid nitrogen (LN2) and a rapid increase in temperature when the LN2 cooling was completed. Temperature increase by 10°C occurred within 5 hours, while it was projected to last 48 hours.
As the Covid-19 pandemic gradually faded during the project’s lifetime, the funding company withdrew from funding the development and covering the cost of the research. Work on the construction of the prototype was interrupted.
It is worth noting that one device for obtaining liquid nitrogen from the air (Figure 1) with an electrical power consumption of 13 kW is able to „power” more than 20 individual devices for storing vaccines. Such energy efficiency cannot be achieved with devices known on the market. A big advantage of this device is its neutrality for the environment and its operation does not require specialist service.
This solution is in line with the implementation of Goals 3 and 13 of the United Nations Resolutions of 25 September 2015 in New York, a resolution signed by all 193 UN member states.- figure 6.
 Figure 6 . Resolution adopted by the General Assembly of the United Nations on w5 September 2015.
Figure 6 . Resolution adopted by the General Assembly of the United Nations on w5 September 2015.
I am looking for a company interested in continuing development work on an innovative device as part of its business. I will also be grateful for any comments.
Gdansk, Poland 25.05.2024 Phd, Eng. Gregor (Grzegorz) MIZERA
